
Teknologi inovatif dengan double air matching filter





The Noble menampilkan desain pemenang penghargaan dalam warna Pebble Grey yang harmonis dan motif alami yang cocok untuk hampir semua ruang.


Pilih pengaturan sesuai dengan keinginan dengan hanya satu sentuhan.
Otomatis membersihkan udara sesuai sensor tingkat polusi dan menyesuaikan arah angin secara real time


Warna lampu indikator LED memberikan notifikasi langsung akanlevel polusi udara sekitar
Roda tersembunyi terpasang di bagian bawah untuk memudahkan Anda memindahkan Air Purifier kemana saja.


Menyaring partikel besar seperti rambut, bulu, pasir dan debu

Menyaring gas berbahaya seperti Nitrogen Dioxide (NO2) secara efektif

Mengurangi tungau debu serta alergen anjing dan kucing

Menghilangkan bau tidak sedap dan gas berbahaya

Menonaktifkan SARS COV-2 varian omicron, Influenza A Virus (H1N1), HumanCoronavirus (HCoV OC43) hingga 99,999%

Menonaktifkan bakteria seperti E.Coli hingga 99,9%
Model
NOBLE (AP-2021A)
fILTRATION sYSTEM
hepa fILTER sYSTEM
Filter | Masa Filter
4D Pre-Filter | Dapat dicuci
Air Matching Filter | 4 Bulan
Double HEPA Filter | 12 bulan
Deodorisation Filter | 24 bulan
Ukuran Ruang
66 m2
CADR
7,4 m3/min (444 m3/jam)
Deodorization Rate
99.0%
Noise
Under 50 dB
Konsumsi Listrik
55Watt
Nilai Tegangan / Frekuensi
220V – 240V / 50Hz
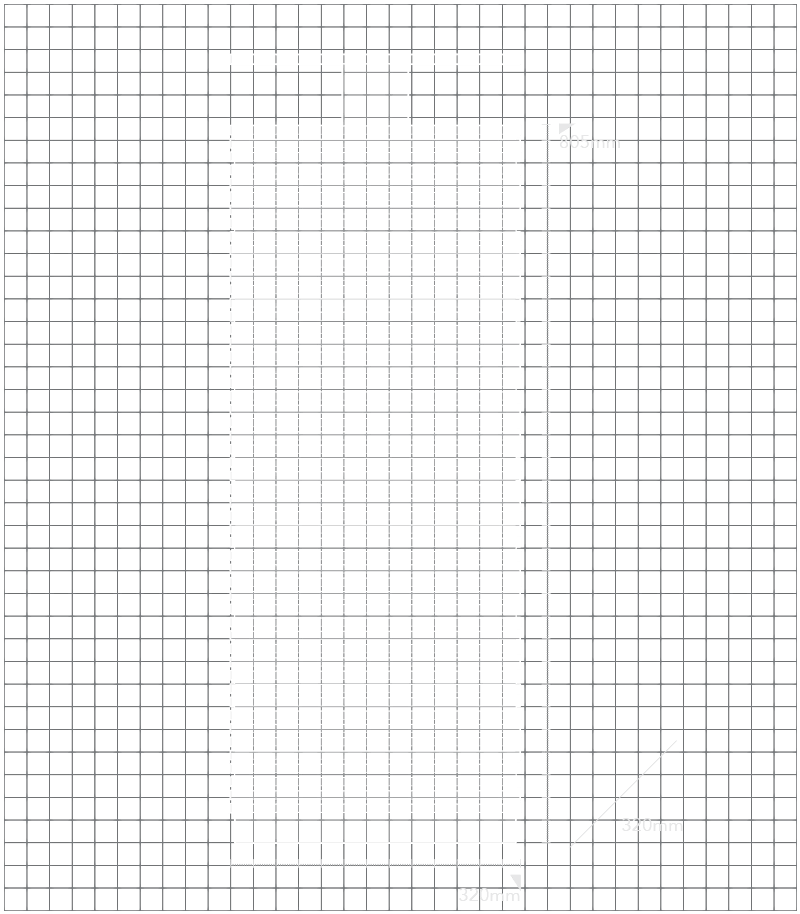
Dimensi
320 x 320 x 805 mm
Berat Bersih
15 kg
Instalation Are
Indoor
Spesifications are subject to change without prior notice
Disclaimer:
1. Advanced 6-Step Filtration removes up to 99.999% Ultrafine Dust and 99.9% Airborne viruses, bacteria, black mold.
2. Double HEPA with advanced filter material inactivates up to 99.999% of SARS-CoV-2 Omicron Variant, Influenza A virus (H1N1), Human Coronavirus (HCoV-OC43) with its antibacterial and antifungal performance.
(Gratis perawatan unit, penggantian fiter dan garansi unit hingga 84 bulan)
(Gratis perawatan unit, penggantian fiter dan garansi unit hingga 72 bulan)
(Gratis perawatan unit, penggantian fiter dan garansi unit hingga 60 bulan)
(Gratis perawatan unit, penggantian fiter dan garansi unit hingga 36 bulan)
(Tanpa perawatan unit, penggantian fiter dan garansi unit hingga 12 bulan)
(Perawatan unit dan penggantian fiter untuk 12 bulan)
Harga produk Coway sudah termasuk pajak yang berlaku, biaya pengiriman dan instalasi didalam area sevice (Jakarta, Tangerang, Serang, Bekasi, Depok, Bogor, Bandung, Garut, Semarang, Surabaya, Medan). Garansi unit hanya meliputi kerusakan dengan penggunaan atau pemakaian normal, kerusakan yang diakibatkan kelalaian pengguna tidak termasuk dalam garansi yang dimaksud

J. Edwin
Pro Health Planner
Hai, tertarik untuk lebih detil tentang produk dan promo Coway